
Ivor Smajlagic, Matt Guest, Rocío Durán, Barbara Herrera, Travis Dudding.This article is cited by 23 publications. A calculated difference density plot showed that little electronic reorganization occurs at the nitrile functional group of DMAAN during rotation about the C−N bond. The estimate for the gas-phase barrier of DMAAN obtained from this correlation agreed closely with that derived earlier on the basis of the relationship with the DMA barriers. For a subset of solvents that were nonaromatic and non-chlorinated as well as aprotic, the barrier correlated closely with the Onsager dielectric function, (ε − 1)/(2ε + 1). For aprotic solvents, the solvent effects were linearly related to Brownstein's empirical solvent polarity parameter S. High-level ab initio calculations yielded good agreement with the gas-phase barrier extrapolated from the experimental data. Comparison of the solvent dependence of the barriers in these two compounds was used to estimate the gas-phase barrier in DMAAN at 9.3 kcal/mol. For aprotic solvents, the variation of the DMAAN barrier with solvent correlated closely with the solvent dependence previously observed for the rotational barriers of dimethylformamide (DMF) and dimethylacetamide (DMA). In striking contrast to amides, however, the barrier was found not to depend on solvent hydrogen bond donor ability. The barrier was found to increase with solvent polarity, as is the case for amides. Generally, solvents with dielectric constants greater than about 5 are considered "polar" and those with dielectric constants less than 5 are considered "non-polar.The barrier to rotation about the conjugated C−N bond of N, N-dimethylaminoacrylonitrile (DMAAN) was determined by dynamic NMR spectroscopy in the solvents methylcyclohexane, dibutyl ether, toluene, dichloromethane, chloroform, acetone, acetonitrile, nitromethane, methanol, and water.

However, as with many properties, the polarity is a continuous scale, and the correct question is not "is it polar or non-polar" but "how polar is it." Nonetheless, guidelines have been created to make it easier. Solvents are generally classified by the polarity, and considered either polar or non-polar, as indicated by the dielectric constant. Because non-polar solvents tend to be aprotic,the focus is upon polar solvents and their structures. Among the most important are whether the solvents are polar or non-polar, and whether they are protic or aprotic.
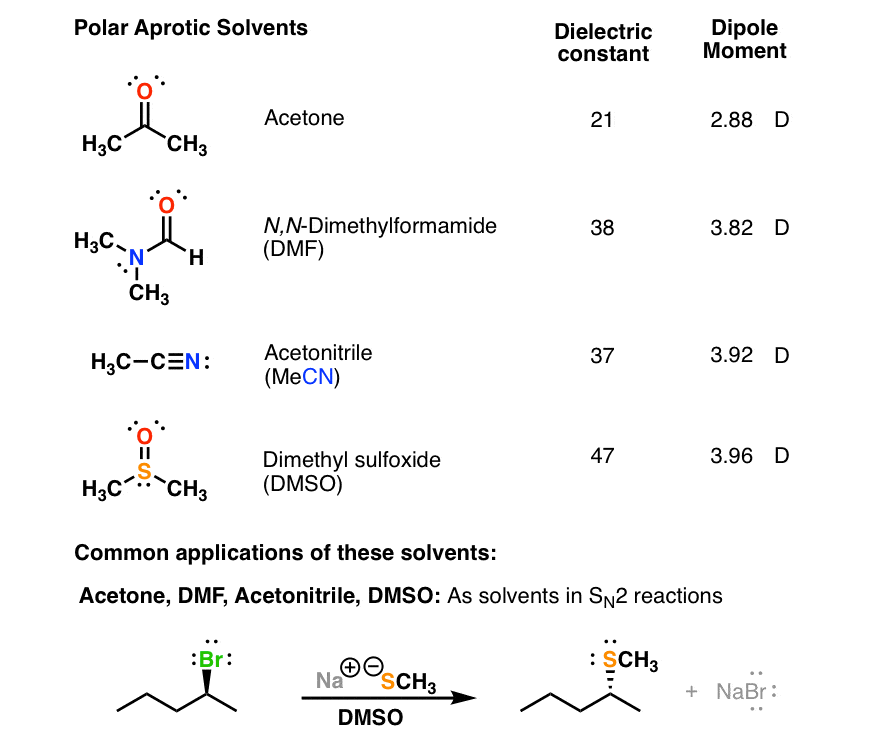
Solvents used in organic chemistry are characterized by their physical characteristics.


 0 kommentar(er)
0 kommentar(er)
